


This page introduces some of the basic concepts behind climate and global warming including: the energy flow to and from the Earth; the greenhouse effect and how carbon dioxide accumulation leads to global warming. The next 3 pages (climate change 1, 2, 3) go on to discuss evidence for global warming, whether it's our fault and some of the predicted effects of climate change.
To quote from the excellent book by Prof. Richard Turco, "The climate system can be thought of as a gigantic machine, whose parts consist of every molecule of the atmosphere, oceans, and land, and every living organism that walks, flies, swims, or crawls" (Turco 2002). This gives us some idea of the enormous complexity of the system.
But for general use the climate can be defined as the long term average of the weather experienced at any location, which may be as small as a city, up to the scale of the whole planet. The most stable indicator of the climate is the global average temperature, as this balances out the influences of the day/night cycle, seasons and other variations in local weather. Global average temperature is an indicator of the total energy from the Sun absorbed and stored by the Earth. The oceans, land and atmosphere - the so-called reservoirs, absorb and store heat for varying lengths of time, helping to smooth out temperature variations. The global average temperature has not varied by more than a degree for centuries, or millenia.
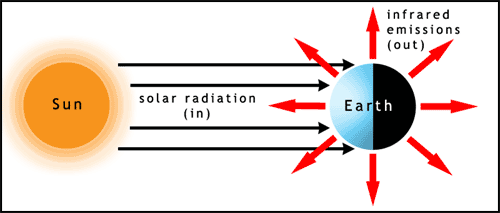
The first factor to consider in relation to the climate is the Sun. Almost all of the energy input to Earth comes from sunlight. The light hits the Earth at different angles on different parts of the planet. The poles receive sunlight at a low angle of incidence (the Sun appears to be low in the sky, when it's visible at all), this spreads the energy over a larger surface area and therefore reduces the heating per unit area. The Earth radiates large amounts of energy back into space as infrared light (figure 1). For some information about light visit the photosynthesis page.
You can try this for yourself, shine a torch (flashlight) on a piece of paper to produce a round spot of light, then tilt the paper and see how the light spreads out.
The Earth experiences seasons due to its slight axial tilt (figure 2). As the planet orbits the Sun this tilt results in one hemisphere getting more sunlight (a higher angle of incidence, the Sun appears higher in the sky) in one part of the year, causing summer. Winter occurs 6 months later due to the pole being tilted away from the Sun, therefore receiving less energy. This tilt leads to the southern and northern hemispheres having opposite seasons in the same months.
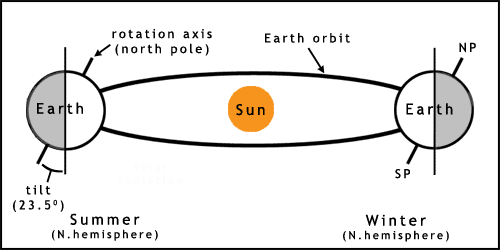
The orbit of the Earth is not quite circular, which also leads to variation in climate. This and other factors may lead to longer term changes such as ice ages. For more information on more complex aspects of solar/earth cycles see the links and references.
The Sun continually supplies energy to the Earth, so why doesn't the planet overheat rapidly? Because the same amount of energy is radiated back out into space by the Earth as infrared light. However the Earth has some average temperature above absolute zero. This is due to heat being stored in a variety of reservoirs (oceans, land, atmosphere). Using a range of data and equations the effective (average) temperature of the Earth can be calculated. This is predicted to be -18oC (255 kelvin). However, luckily for us, the actual average temperature of the land and oceans is about +17oC (290 K). Why the difference? The answer is the greenhouse effect.
The Earth's atmosphere contains a range of molecules that absorb light at many different wavelengths. Most of the ultraviolet in sunlight is absorbed by oxygen (O2) and ozone (O3) before it reaches the Earth's surface. Similarly, much of the infrared that is emitted by the Earth is absorbed by water vapour (H2O) and carbon dioxide (CO2).
There are 2 so-called atmospheric windows (figure 3):
These energy flows balance to maintain the Earth's temperature.
BUT, if molecules are added to the atmosphere that block either of the windows then the energy balance will change, leading to temperature changes on the Earth. Many of these molecules, such as CO2, methane, nitrous oxide and chlorofluorocarbons (CFCs) are called collectively greenhouse gases because they block infrared emissions from leaving the Earth.
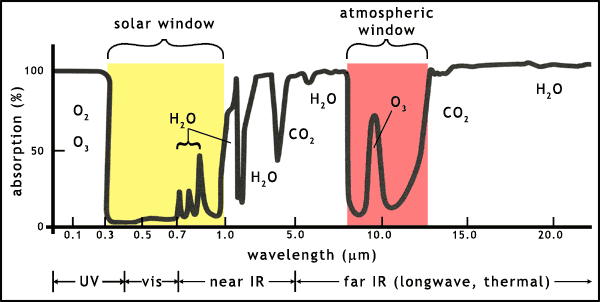
Carbon dioxide is a molecule that can block infrared emissions into space. As shown in figure 3, CO2 and water vapour already block large parts of the Earth's thermal emissions, raising the average temperature of the planet to a level habitable for higher lifeforms. But the addition of more CO2 increases this greenhouse effect. The planet Venus provides us with a fine example of an extreme greenhouse effect. Using the same types of equation on the energy balance of Venus as used on the Earth leads to a theoretical average global temperature of 27oC (300 K), but the vast amount of CO2 in the atmosphere of Venus has led to a temperature of 427oC (700 K). Obviously this is a rather extreme example of the greenhouse effect!
Water vapour is actually the most powerful greenhouse gas, but is not generally called a greenhouse gas due to its emission being largely out of human control. Clouds control most of the Earth's reflectivity (albedo), and reduce the energy reaching the Earth's surface. We might think that clouds could be a global climate thermostat, regulating the Earth's temperature. For instance, if the climate heats up, more water is evaporated producing more clouds, which reflect more of the sunlight into space. To some extent this is true, but clouds are very complex and their influence depends on altitude.
After expert climatologists have taken a variety of factors into account, it turns out that water vapour and clouds are significant positive contributers to the greenhouse effect and they may also enhance the warming caused by other greenhouse gases (Turco 2002).
Methane (CH4) is a greenhouse gas that is 23 times more potent than CO2. Large amounts of this gas are released from human activities, especially agriculture. Livestock emit large quantities of this gas. Methane causes about one third of the warming of CO2 i.e. radiative forcing of around 0.5 W/m2 (CH4) and 1.5 W/m2 (CO2). For more information on radiative forcing click here.
Carbon is found in a variety of forms in all of the so-called reservoirs on Earth: atmosphere, ocean, ocean sediments and land. It is one of the key elements of life on Earth. The total mass of carbon on Earth is about 7 × 1016 tonnes (for a note on exponents click here, opens in a new window).
Carbon remains in these reservoirs for varying lengths of time before being cycled. Table 1 shows the amounts of carbon, the form and the amount turned over per year for each of the major reservoirs.
| Reservoir (C form) |
Amount (tonnes) |
Turnover (tonnes/yr) |
|---|---|---|
| atmosphere (CO2) |
7.5 x 1011 | 8 x 1010 |
| oceans (HCO3-) |
4 x 1013 | 2.5 x 108 |
| sediments (CO32-) |
6 x 1016 | 2.5 x 108 |
| land (organic) |
8 x 1011 (living) 2 x 1012 (dead) |
8 x 1010 (living) 8 x 1010 (dead) |
A range of processes turnover the carbon in these reservoirs over very varying timescales. The geologic carbon cycle, that involves the dissolving of CO2 in the oceans, the sedimentation of carbonates, the formation of sedimentary rocks and finally CO2 emission from volcanoes, takes place over hundreds of millions of years. The time to turnover the sedimentary reservoir is around 240 million years.
The atmosphere/land carbon cycle is of particular interest to us. The CO2 in the atmosphere is used by plants in photosynthesis, these in turn support the food chains. All of the biologically important molecules (except water) use carbon to form their core structures, and the human body is about 18% carbon by mass. Plants are almost totally composed of atoms from CO2 and water. These together with the energy from sunlight allow our whole ecosystem to exist. Roughly the same amount of carbon that enters the living biomass every year, also leaves it to form dead biomass. This is decomposed by a range of specialised organisms to eventually release the carbon back into the atmosphere as CO2. About 10% of the atmospheric CO2 is turned over per year, of this around 99.9% is due to photosynthesis, with the remainder being other processes e.g. dissolving into the oceans.
This is where human CO2 producing activities fit in. Fossil fuels consist of the preserved, transformed remains of plants and animals that died hundreds of millions of years ago. By burning these, we release large amounts of CO2 into the atmosphere. As we saw earlier, this extra CO2 helps to block the atmospheric window, reducing the infrared emissions into space and therefore leading to the slow increase in the average global temperature.
But, we know that plants use CO2, so will they respond to the increased CO2 levels by growing more, thereby balancing out the increases? It seems that the answer is no (more or less). Writing in the journal Science, researchers from Stanford University concluded that elevated atmospheric CO2 actually reduces plant growth when combined with other likely consequences of climate change namely, higher temperatures, increased precipitation or increased nitrogen deposits in the soil. The biggest surprise from the study was the discovery that elevated CO2 only stimulated plant growth when nitrogen, water and temperature were kept at normal levels. We therefore can't just rely on plants growing more and balancing out the increased CO2 levels.
The next pages (climate change 1, 2 and 3) explore some of the evidence for climate change, if these changes are due to human activities and what the potential effects of these changes might be.
