


This page introduces the process of photosynthesis, one of the most important biological processes on Earth. The plant structures involved are described and also some of the molecules of particular relevance to our project. We also briefly describe some features of light.
Photosynthesis is the process by which plants, algae (and certain types of bacteria) use sunlight to convert inorganic components (mostly carbon dioxide and water) into sugar molecules. They then use these molecules to build further organic molecules that make the plants' structures, as well as providing chemical energy.
Photosynthesis is at the start of 99% of the food chains on Earth and plant materials are used in many other ways, such as fuel and as building materials. Many animals eat plants to obtain energy and matter, and in turn these animals are often eaten by others. Ultimately the Sun provides all of the metabolic energy on planet Earth (except for some very small, specialised food chains in the deep oceans around hydrothermal vents).
The key chemical pathway in photosynthesis is the conversion of carbon dioxide (CO2 from the air) and water (H2O) into carbohydrate molecules ([CH2O]n such as sugar) and free oxygen (O2), using light as an energy source for the reactions, as shown here:
the basic form:
the more chemically accurate form:
These carbohydrate molecules, such as sugars, contain more energy than the starting molecules, in other words they act as chemical batteries for solar energy. The carbohydrate molecules are often then used to construct more complex molecules, such as cellulose and lignin, that make the plant structure.
To release stored energy, both plants and animals use oxygen from the atmosphere to convert carbohydrates back into CO2 and H2O. This process is called respiration. However the rate of photosynthesis is usually 30 times the rate of respiration, so plants produce large net quantities of oxygen that is then used by animals that in turn produce CO2 which feeds the plants, in a cycle.
Leaves are the structures in plants that have evolved primarily to absorb sunlight and to convert this energy into carbohydrates. Figure 1 shows a cross-section through a typical leaf.
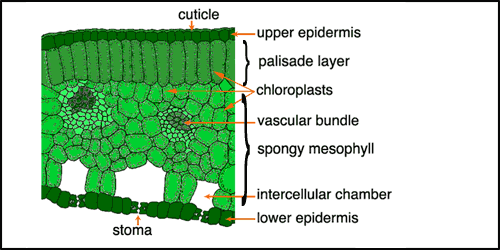
Figure 2 shows a typical plant cell. The nucleus contains the DNA of the cell that encodes the information that the cell needs to function. Respiration takes place within mitochondria (singular: mitochondrion). The vacuole is a fluid-filled bag that often takes up more than 80% of plant cell volume. The major role of vacuoles in most plants is to maintain turgor pressure i.e. to keep the cell rigid, thus helping to support the plant, along with the cell wall (notice how many plants wilt when they lack water).
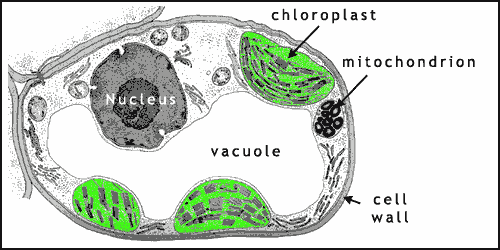
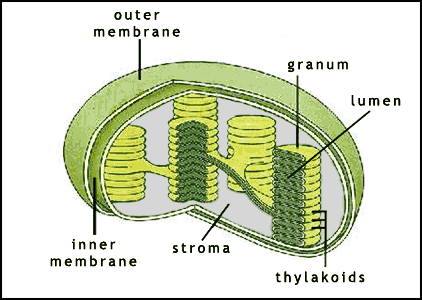
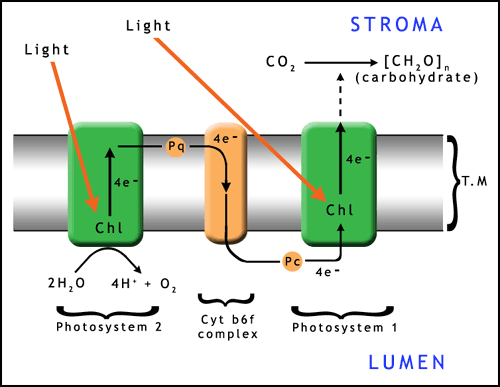
Photosynthesis takes place within chloroplasts (figures 2 and 3) contained within the green cells of plants and algae (photosynthesizing bacteria have other, simpler structures). Chloroplasts are flattened disc shaped organelles usually 2-10 μm in diameter and 1 μm thick (click here to open a note on units in a new window).
The chloroplast has a two membrane envelope termed the inner and outer membranes, with a space inbetween. The fluid within the chloroplast is called the stroma. Within the stroma are stacks of thylakoids, the sub-organelles where photosynthesis actually takes place. A stack of thylakoids is called a granum (plural: grana). A thylakoid looks like a flattened disk, and inside is the thylakoid space or lumen. The photosynthetic reaction takes place on the thylakoid membrane.
Before introducing the photosynthetic molecules, we will briefly discuss light. What we call "light" is actually part of the spectrum of electromagnetic radiation. This covers a range of rays or waves from cosmic rays, with very short wavelengths all the way to radio waves (figure 4).
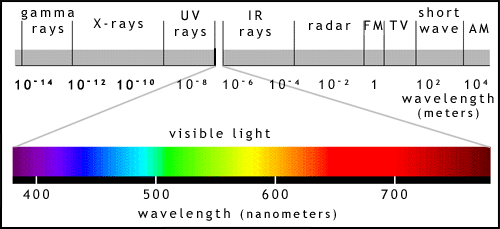
The wavelength is the distance between the peaks of 2 adjacent waves (figure 5). Rather confusingly, light has some properties of waves and some of particles. A light particle is called a photon. In some situations it is more convenient to think of light as photons, and in others to treat it like waves.
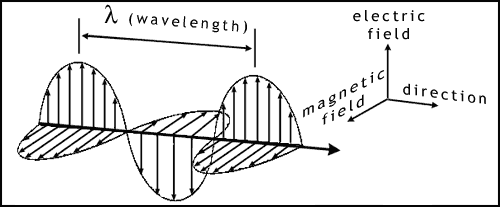
There is a direct relationship between wavelength, frequency and the speed of light: v = f λ
The speed of light doesn't vary, so the longer the wavelength the smaller the frequency.
There is also a relationship between frequency and the energy of each wavelength of light: E = hf
The higher frequency (shorter wavelength) light has more energy. When calculated, the energy levels are as shown in table 1. You can see that red light has considerably less energy than blue light.
| λ | Colour | Energy |
|---|---|---|
| 700nm | red | 1.7 x 105 |
| 600nm | yellow | 2.0 x 105 |
| 500nm | blue | 2.4 x 105 |
| 400nm | violet | 3.0 x 105 |
An example that shows the difference in energies of the colours of light is seen on older cars (automobiles). Red cars absorb the higher energy blue photons, but reflect the lower energy red photons, that's why the paint looks red. This absorption of more energetic photons leads to faster oxidation of the paintwork.
Photons with high enough energy can be absorbed by particular molecules in the plant, producing energised electrons. It is important to note that 1 photon can only supply its energy to 1 electron and also that photons with too low energy will not be used in photosynthesis. The red photons have less energy than the blue photons, this explains why infrared light is relatively poorly used in photosynthesis.
The absorption of light in chloroplasts is by a range of pigments, mainly chlorophylls (which provide the green colour) and carotenoids. These different types of pigment absorb light of different wavelengths. A large number of these pigment molecules are arranged into an antenna to capture most of the photons of the correct energy. This energy is then passed through a chain of pigment molecules until it reaches a specialised molecular complex called the reaction centre. The pigment antenna complex/reaction centre together are called a photosystem (figure 6).
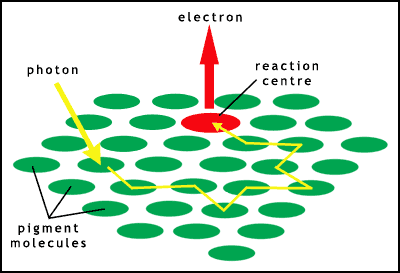
There are 2 types of photosystem in plants, linked together in a 2 stage process. Light is absorbed at each of the photosystems to provide enough energy to manufacture carbohydrates (figure 7). There are millions of these photosystems embedded in the thylakoid membranes within the chloroplasts.
For more detail I recommend Photosynthesis (Hall and Rao, 1999). This is an excellent introduction to the subject at an undergraduate level. For a general grounding in cell biology I recommend the book Molecular Biology of the Cell, (Alberts et al. 2001) which also has an introduction to photosynthesis.
The scale of the processes of photosynthesis and respiration is quite staggering. For instance around 10,000 tonnes of oxygen is consumed every second by respiration and the burning of fuels. If plants didn't balance this loss by their production, oxygen supplies in the atmosphere would only last about 3000 years. The amount of plant biomass produced every year has been estimated at 200 billion tonnes.
How much solar energy is absorbed by photosynthesis, or what percentage of sunlight falling on the Earth's surface is converted into plant matter?
The sunlight hitting the atmosphere every year is about 5.6 trillion trillion Joules, or 5.6 x 1024 J (a note on units and exponents is here, opens in a new window). This is equivalent to a continual flow of 1.8 x 1017 Watts. About half of this reaches the Earth's surface, the rest being reflected by the atmosphere and clouds. Of this 50%, some is reflected from the oceans and other surfaces leaving 30%. But only about half of this light is of the correct wavelength to enable photosynthesis. This leaves 15% of the original sunlight available for absorption by plants (8.4 x 1023 J).
Assuming the primary biomass production (plants) of Earth is 2 x 1011 tonnes per year, this equates to about 3 x 1021 J of energy stored as biomass.
Therefore the percentage of usable light stored by photosynthesis globally is: 0.4%
So why the difference in figures?
The measured efficiencies of energy conversion (i.e. sunlight into biomass) for various plants range from as low as 0.1%, to around 1.5% (most crop plants) to sugarcane at 8% (the exception). The reasons for this are complex, and range from the behaviour of light energy as it penetrates leaves, to limiting factors in the plant's environment e.g. temperature, micronutrients etc. There is also much room for error in calculating such enormous processes.
If plants could be made more efficient at converting light into plant matter than the productivity of the world's agriculture could be improved vastly. Numerous research projects are focusing on achieving this with a variety of plant species.
